

But that makes no difference to the discussion.įor comparison purposes later, I need to work out the lead(II) ion concentration in this saturated solution. There are serious discrepancies between the values from different sources. Note: I have no confidence in the accuracy of this value. The expression for the solubility product and its value are given by: If you just shook up some solid lead(II) chloride with water, then the solution would obviously contain twice as many chloride ions as lead(II) ions. Lead(II) chloride is sparingly soluble in water, and this equilibrium is set up between the solid and its ions in solution: The solubility of lead(II) chloride in water
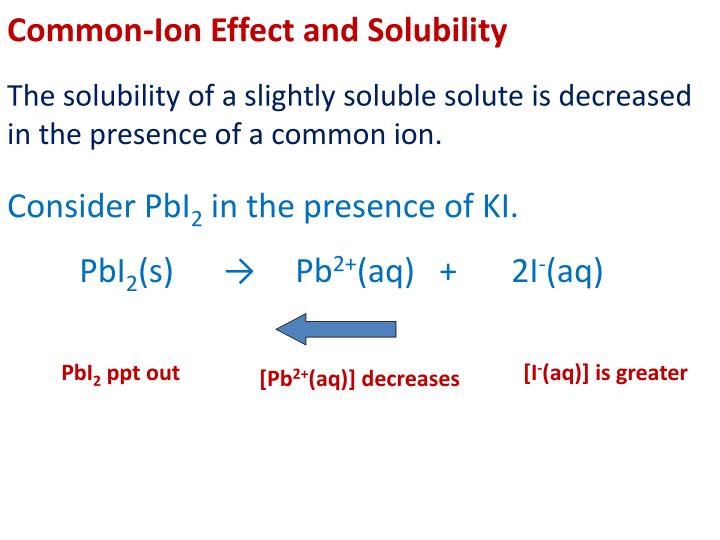
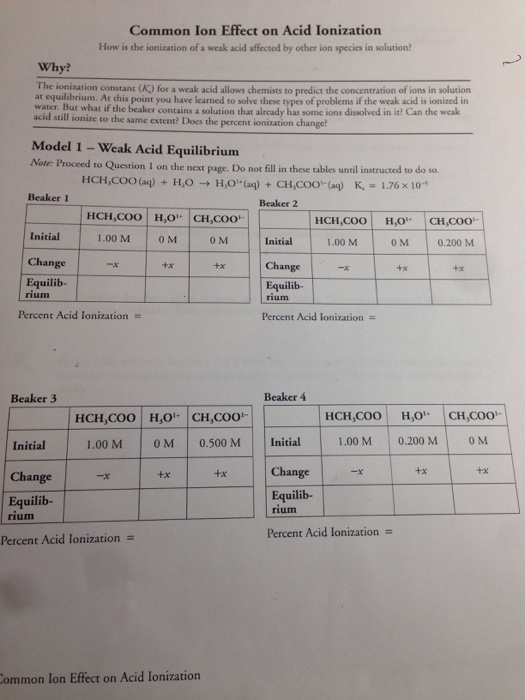
I need to look again at a simple solubility product calculation, before we go on to the common ion effect. You need to know about solubility products and calculations involving them before you read this page. This page looks at the common ion effect related to solubility products, including a simple calculation. SOLUBILITY PRODUCT and THE COMMON ION EFFECT


 0 kommentar(er)
0 kommentar(er)
